Electrospinning of Core-Shell Fibers
for Drug Release Systems
Dennis Pedersbæk, Magnus Tudsborg Frantzen and Peter Fojan*
Department of Physics and Nanotechnology, Aalborg University
Skjernvej 4A, 9220 Aalborg Ø, Denmark
*Corresponding Author: fp@nano.aau.dk
Received 7 January 2017; Accepted 24 March 2017;
Publication 22 April 2017
Abstract
Electrospinning is a promising method for the fabrication of fibers used for drug release system, due the ease of operation of the electrospinning process and the high surface to volume ratio, high loading capacity and high encapsulation efficiency of the obtained fibers. In this study release of tetracycline hydrochloride (TCH) from both monolithic and core-shell electrospun fibers of poly-lactic acid (PLA), poly-ϵ-caprolactone (PCL) and their blend were studied. It was found that the drug release from the fibers depended on their composition. Core-shell fibers were designed with PCL and TCH in the core and with blends (PCL/PLA) of varying composition in the shell. A varying initial burst release was observed when the composition of the shell was varied. This illustrates that the burst release might be tunable which can be advantageous for many applications.
Keywords
- Electrospinning
- core-shell fibers
- drug release
- burst release
- poly-ϵ-caprolactone
- poly-lactic acid
1 Introduction
Electrospinning is a simple method which can be used to fabricate ultrathin fibers from a variety of materials [1, 2]. The textile industry has been using electrospinning for many years to produce non-woven fiber fabrics [2]. A renewed interest in the method has aspired due to applications of the fibers in areas such as filtration, optical and chemical sensors and electrode materials [2]. Recently, also biomedical applications of the fibers have been researched, e.g. the fibers can be used for scaffolds for tissue engineering [3] and drug delivery of various substances [4 – 6].
Advantages of using electrospun fibers for drug release systems include the high surface to volume ratio of the fibers and the high loading capacity of drug in the fibers [3, 5]. Many different drugs have been incorporated into electrospun fibers, which include antimicrobial, anticancer drug and bioactive molecules such as protein, DNA, RNA and growth factors [5, 6]. Polymers are widely applied as potential candidates for the encapsulation of drug for drug delivery applications, e.g. in micelles [7 – 9]. The polymers can be both biodegradable and biocompatible which makes them ideal for biomedical applications [10], thus using similar polymers for the electrospun fibers seems promising. Examples of such polymers used for drug delivery from electrospun fibers include amongst many others [5] PLA [4, 11] and PCL [10].
In the standard electrospinning process a polymer solution is supplied through a needle and a high voltage is applied to the needle [1, 2]. The resulting electrostatic field arising between the needle and collector plate draws a thin jet from the droplet at the tip of the needle [1, 2]. The jet is initially drawn linearly towards the collector but will eventually begin a whipping process during which the solvent will evaporate, and a solid non-woven fiber mat will be obtained on the collector [1, 2, 5]. Many parameters of the electrospinning process can be varied which can affect the morphology of the obtained fibers [1, 2]. In order to convert these monolithic fibers into a drug release system, drugs can simply be added to the polymer solution [10]. The drug can subsequently be released by diffusion out of the polymeric matrix [6, 10 – 12] or the degradation of the polymers can be utilized [4, 13]. Many parameters can influence the release profiles of the drug, e.g. fiber morphology or drug concentration [6].
Several modifications of the standard electrospinning process have been developed, amongst these are the coaxial electrospinning where the nozzle consists of a smaller capillary inside and coaxial to a larger capillary [3]. Core-shell fibers are obtained, and the method obviously allows for supply of separate solutions for the core and the shell [2, 3]. Hence, it is possible to design multi-drug delivery systems using core-shell fibers [14]. If only the core contains drug, the shell can function as a diffusion barrier which also protects the core from undesirable interactions between drug and the environment [5].
In the present study, the release of the antibiotic TCH from both monolithic fibers and core-shell fibers will be investigated. Applications of drug release systems containing antibiotics include for example wound healing applications [5, 6]. The monolithic fibers investigated in this study consisted of PLA, PCL and blends thereof. The core-shell fibers had a core consisting of PCL and contained the TCH, while the shell consisted of blends of PLA and PCL in various ratios in order to investigate the dependence of the PLA:PCL ratio on TCH release from the fiber core.
2 Experimental Procedures
2.1 Materials
The used d,l-PLA was obtained from MakerBot (Germany). The density of the PLA was 1.25 g/cm3. PCL was Capa 6506 from Perstorp UK Limited. The PCL had an average molecular weight of 50,000 kDa. Both polymers were dissolved in chloroform (≥ 99.8%). TCH (≥ 95%) was dissolved in methanol (≥ 99.6%) prior to the addition to the polymer solution. Chloroform, methanol and TCH were purchased from Sigma-Aldrich (Denmark). All polymers and solvents have been used as supplied without further purification.
2.2 Solutions
Multiple polymer stock solutions were prepared with different concentration and polymer type. For the monolithic fibers the concentrations were 5 wt.%, 15 wt.% and 12.5 wt.% for the PLA, PCL and blend solutions, respectively. The concentration of the blend solution denotes the amount of total polymer. For the core-shell fibers the concentrations were 10 wt.% and 12.5 wt.% for the core (PCL) and shell (blend), respectively. However, the shell of pure PLA had to be electrospun from a solution of 10 wt.% due to too high viscosity if the concentration was increased further. Prior to the electrospinning process the solutions were prepared by mixing the polymer stock solution and the solution containing TCH such that the final solvent ratio of chloroform/methanol was 19:1. It was ensured that the final concentration of TCH was kept at 5 wt.% relative to the polymer by varying the TCH concentration in the methanol solution.
2.3 Electrospinning Procedure
The electrospinning was done using an Electrospinner 2.2.D-500 from YFlow Nanotechnology Solutions (Spain). The needle used for electrospinning of the monolithic fibers had a diameter of 1 mm, and the needle used for electrospinning of the core-shell fibers consisted of a needle with a diameter of 2.5 mm with an 1 mm inner capillary. The voltage at the needle and the collector could be adjusted separately. The voltage at the needle is denoted injector voltage. The voltage at the collector was kept at –5 kV while the injector voltage was varied between experiments. A camera was attached to the set-up for some of the experiments to observe the tip of the needle. It was not possible to obtain a stable electrospinning process if the voltage was kept constant when the composition of the polymer solutions was changed. Therefore, the injector voltage was changed but kept in the range 12.5 kV – 17.0 kV for electrospinning of the monolithic fibers and 6.8 kV – 13.6 kV for the core-shell fibers. The fibers were collected on glass wafers.
2.4 Characterization
The fibers were characterized using a scanning electron microscope (SEM) (Zeiss EVO 60 SEM system from Zeiss), an optical microscopy (Axioskop 2 plus) and a fluorescence microscope (Olympus IX71) with cube U-MWU2 fluorescence filter (330–385 nm /420 nm). SEM was used to characterize the fiber morphology, and the diameter of the fibers was estimated by the obtained images using ImageJ software.
2.5 Release Measurements
The fibers was submerged into a phosphate-buffered saline (PBS) solution for the release measurements. The release of TCH was followed by measuring the absorption at 360 nm by a UV-1800 spectrophotometer from SHIMADZU. For each absorption measurement 1 mL was removed from the solution containing the fibers and replaced with 1 mL fresh PBS solution in order to ensure sink conditions.
3 Results and Discussion
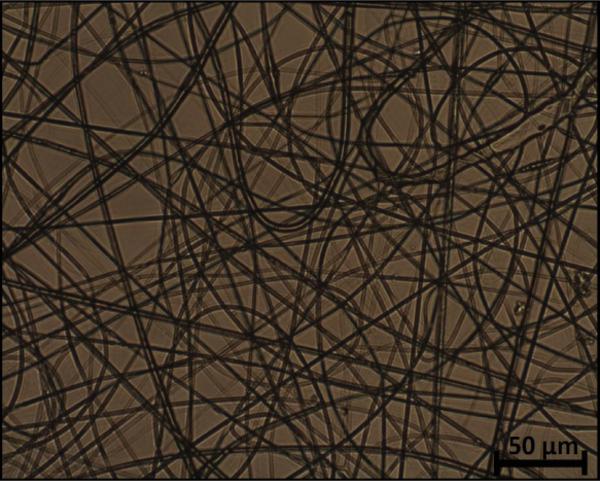
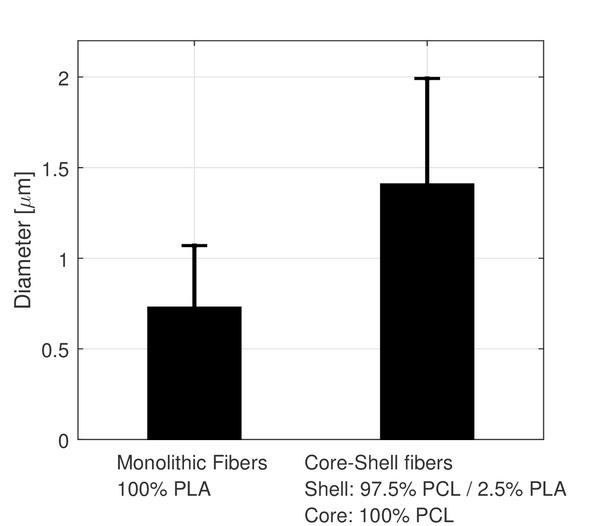
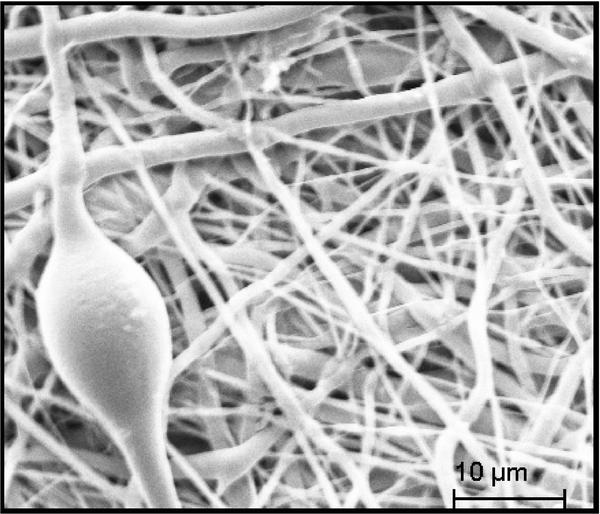
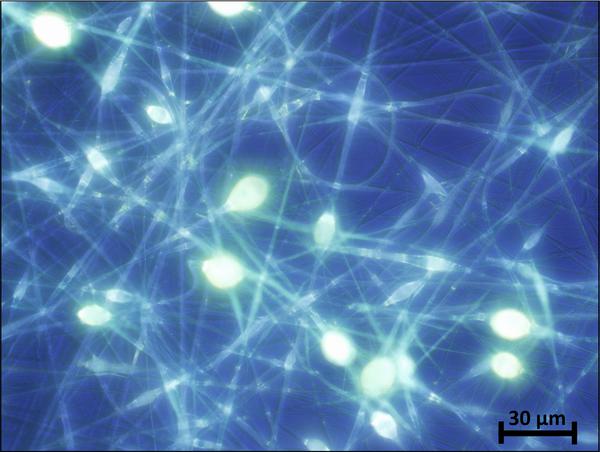
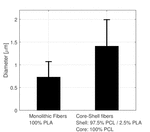
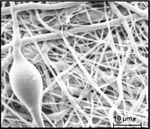
The electrospun fibers formed a fiber mat on the collector plate. This could be visually seen both by the naked eye and light microscopy (Figure 1). The core-shell fibers seemed to have a larger mean diameter than the monolithic fibers, but it was also evident that the standard deviation of the fiber diameter distribution was relatively wide (Figure 2). The larger diameter of the core-shell fibers might be explained by the larger diameter of the needle which consequently supplies more polymer solution than when electrospinning monolithic fibers under similar conditions. However, in order to obtain core-shell fibers different parameters than used for the electrospinning of the monolithic fibers had to be used, e.g. different voltage and concentration of the polymer solution. These varying parameters could potentially also affect the diameter of the fibers. The large standard deviation of the core-shell fibers is immediately evident from the SEM images (Figure 3). Furthermore, beads were observed, especially when high amounts of PCL in the blend solution were used. These factors indicate that the electrospinning parameters could be optimized further, however, each variation of the polymer composition would require re-optimization of the electrospinning parameters. Hence, this study focused on keeping as many electrospinning parameters as possible constant while varying the composition of the polymer solution within the possible limits of the predetermined parameters. From the fluorescence microscope image of the core-shell fibers, it is evident that TCH must be incorporated into the fibers and relatively evenly distributed (Figure 4). The intensity of the beads is clearly higher, possibly due to the larger volume of the beads compared to the fibers, thus resulting in a higher fluorescence intensity.
Figure 1 Optical microscopic image of monolithic PLA fibers electrospun from a 5 wt.% polymer solution.
Figure 2 Fiber diameter distribution of monolithic and core-shell fibers electrospun from 5 wt.% and 12.5 wt.% polymer solutions, respectively.
Figure 3 Representative SEM image of core-shell fiber electrospun with 1% PLA in the shell and 99% PCL. One of the beads is seen along with fibers of significantly varying diameters.
Figure 4 Fluorescence microscopy image of core-shell fibers with TCH incorporated in the core.
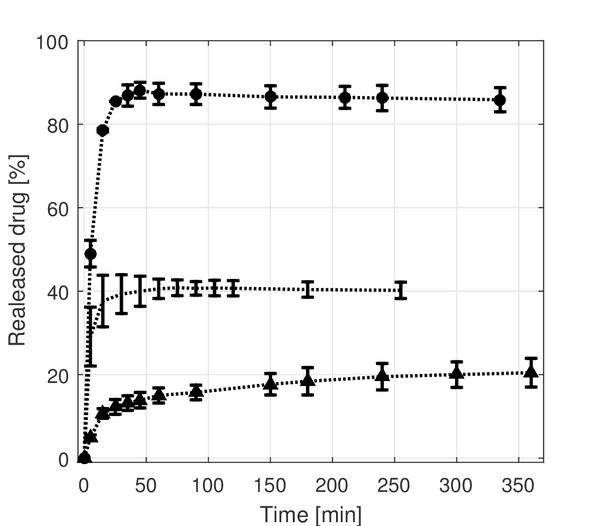
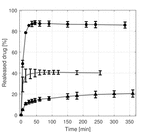
The measured release profiles from the monolithic fibers showed that only 40% of TCH is released from the PLA fibers in a single burst release (Figure 5). This might be attributed to the TCH located on the surface of the fibers, which further implies that diffusion from the inside of the fibers is limited. This was also found in other studies for l-PLA [4, 11], but it is interesting that it also applies for the d,l-PLA used in this study, since d,l-PLA can be considered amorphous in contrast with the semi-crystalline l-PLA [15]. It might be explained by the fact that the glass transition temperature of d,l-PLA is above the body temperature [15], thus it is in the glassy state which potentially limits the permeability of buffer and drug. Also, it might be difficult for TCH to diffuse through the hydrophobic PLA [5]. Almost all TCH seems to be released from the monolithic PCL fibers in a single initial burst release (Figure 5), indicating that the buffer solution can diffuse freely into the polymeric matrix which allows TCH to diffuse freely out of the fibers. The glass transition for PCL is approximately –60 ∘C [16], hence, the amorphous regions of PCL are in a soft and flexible rubbery state which might increase the permeability of TCH. It has also been found in literature that the surface morphology of PLA and PCL fibers differ, since PLA fibers have a more closed rough surface in contrast to the surface of PCL fibers which contain multiple pores [12]. The monolithic blend fibers yielded a different release profile than observed from the pure PLA and PCL fibers. This is quite interesting but has also been reported in other studies, and applies to other types of polymers as well [11, 17]. Although the burst release is minimized for the blend fibers relative to the fibers consisting of pure PCL and PLA, all release profiles reach a plateau after approximately 350 min, hence, no sustained release is obtained for any of the fibers.
Figure 5 Release of TCH from monolithic electrospun fibers. (●) is for the PCL fibers, (…) is for the PLA fibers and (▲) is for the blend fibers which consist of 67% PCL and 33% PLA.
The mean diameters of the monolithic fibers were estimated to be 0.73 (±0.34)μm, 0.33 (±0.071)μm and 0.31 (±0.13)μm for the PLA, PCL and blend fibers, respectively. The observed larger diameter of the PLA fibers might be caused by the different electrospinning parameters, e.g. applied voltage or viscosity of the electrospinning solution. The fiber diameter might influence the release properties of the fibers, however, the effect on the release profiles caused by varying fiber diameter is not believed to be significant, especially since different release profiles are observed for PCL and blend fibers with comparable diameters.
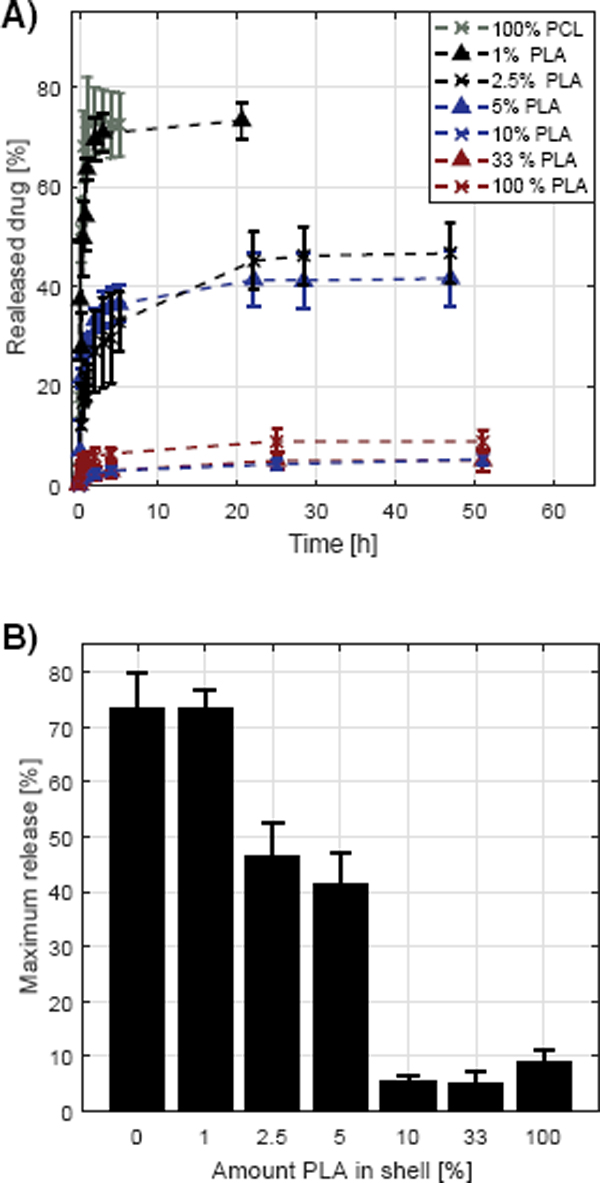
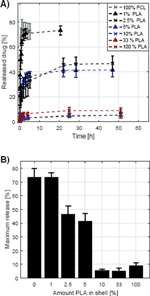
From the release profiles of the core-shell fibers, it is evident that there is almost no release of TCH when the shell contains more than 10% (w/w) PLA in the blend (Figure 6.A). When the shell consists of pure PCL almost all of the drug is released, which illustrates that buffer and thus TCH can diffuse freely through the PCL polymer matrix. On the other hand, TCH does not seem to diffuse through the PLA shell, which illustrates even more clearly that TCH cannot diffuse through the PLA layer. It is interesting that only 10% PLA limits the release of TCH. This might be explained by the fact that PLA and PCL phase separate during the electrospinning process which results in the formation of a layer of PLA surrounding the core which consequently hinders release of TCH. Lowering the amount of PLA in the shell between 10% and 1% results in a varying initial burst release with values between the maximum and minimum burst releases observed (Figure 6.A). The release profile for especially fibers containing 2.5% PLA in the shell seems to increase linearly after the initial burst before reaching a plateau. However, while the difference between the samples in linear release following the burst release might be explained by inhomogeneity between the samples, it is evident that the burst release clearly varies significantly, which most likely is explained by the different compositions of the shell. It is evident that the maximum release, and thus burst release, might be tunable by varying the PCL:PLA ratio in the blend used in the shell (Figure 6.B). An explanation for this phenomenon might be that as the concentration of PLA in the shell is decreased, islands of PLA are formed rather than a complete layer, resulting in some of the core being exposed directly to PCL which results in the release of TCH from these areas. The linear release observed for some samples between the initial burst value and maximum release might be due to TCH diffusing to the exposed areas from underneath the PLA layer.
Figure 6 Release of TCH from core-shell fibers. The shell consisted of a blend of PLA and PCL in various ratios and the core consisted of pure PCL and contained the TCH. (A): The measured release profiles. The legend denotes the amount of PLA in the shell, the rest being PCL. (B): The dependence of maximum release with the amount of PLA in the shell.
The fiber morphology might also affect the release, however, the larger variation in diameter of the core-shell fibers and the varying amount of beads, make it infeasible to conclude on such effects. Since the larger variation in fiber morphology was observed for all the core-shell fibers, it is likely that the fiber composition is the cause of the different release properties of the fibers.
It is evident that no sustained release is obtained over a long period of time for any of the fibers, which would be necessary for a prolonged drug release system, however, this is a first step to obtain a sustained drug release system with a tunable burst release.
Conclusion
Core-shell fibers pose great promise as drug release systems. The fact that the shell and core can be of different composition allows for the design of systems applicable to various applications which would not be possible with monolithic fibers. As it has been shown in this study, the shell can function as a diffusion barrier which can be used to control the drug release from the core, which potentially allows for a tunable burst release. It is necessary for an effective treatment to increase the local drug concentration to the therapeutic range when using drug release systems, hence, a tunable burst release is a major advantage in the design of a novel sustained drug release system.
References
[1] D. Li, and Y. Xia. Electrospinning of Nanofibers: Reinventing the wheel? Adv Mater, 16(14): 1151–1170 (2004).
[2] N. Bhardwaj, and S. C. Kundu. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv, 28: 325–347 (2010).
[3] T. J. Sill and H. A. von Recum. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials, 29:1989–2006, 2008.
[4] J. Zeng, X. Xu, X. Chen, Q. Liang, X. Bian, L. Yang, and X. Jing. Biodegradable electrospun fibers for drug delivery. J Control Release, 92: 227–231 (2003).
[5] Xiuli Hu, S. Liu, G. Zhou, Y. Huang, Z. Xie, and X. Jing. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release, 185:12–21 (2014).
[6] M. Maleki, M. Latifi, M. Amani-Tehran, and S. Mathur. Electrospun core–shell nanofibers for drug encapsulation and sustained release. Polymer Eng. Sci, 53(8): 1770–1779 (2013).
[7] T. M. Allen, and P. R. Cullis. Drug delivery systems: entering the mainstream. Science, 303: 1818–1822 (2004).
[8] R. Langer. Drug delivery and targeting. Nature, 392: 5–10 (1998).
[9] K. Kataoka, A. Harada, and Y. Nagasaki. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev, 64: 37–48 (2012).
[10] J. S. Brown, and R. R. Burton. Processing of polymer nanofibers through electrospinning as drug delivery systems. Mater Chem Phys, 113: 296–302 (2009).
[11] E.-R. Kenawy G. L. Bowlin, K. Mansfield, J. Layman, D. G. Simpson, E. H. Sanders, and Gary E. Wnek. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release, 81: 57–64 (2002).
[12] G. Buschle-Diller, J. Cooper, Z. Xie, Y. Wu, J. Waldrup, and X. Ren. Release of antibiotics from electrospun bicomponent fibers. Cellulose, 14: 553–562 (2007).
[13] K. H. Hong and S. H. Woo, and T. J. Kang. In Vitrodegradation and drug-release behavior of electrospun, fibrous webs of poly(lactic-coglycolic acid). J Appl Polymer Sci, 124: 209–214 (2012).
[14] Y. J. Son, W. J. Kim, and H. S. Yoo. Therapeutic applications of electrospun nanofibers for drug delivery systems. Arch Pharm Res, 37: 69–78 (2014).
[15] T. Tábi, I. E. Sajó, F. Szabó, A. S. Luyt, and J. G. Kovács. Crystalline structure of annealed polylactic acid and its relation to processing. eXPRESS Polymer Lett. 4(10): 659–668 (2010).
[16] M. Labet, and W. Thielemans. Synthesis of polycaprolactone: a review. Chem Soc Rev, 38: 3484–3504 (2009).
[17] M. Jannesari, J. Varshosaz, M. Morshed, and M. Zamani. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int J Nanomed, 6: 993–1003 (2011).
Biographies

D. Pedersbæk received his Bachelor’s degree in Nanotechnology from Aalborg University, Department of Physics and Nanotechnology in 2015. Currently, he is studying Nanobiotechnology at the same department and will intentionally receive his Master’s Degree in 2017. His research interests are focused on nanomedicine and drug delivery systems.

M. Tudsborg Frantzen received his Bachelor’s degree in Nanotechnology from Aalborg University, Department of Physics and Nanotechnology in 2015. Currently, he is pursuing a Master’s Degree in Nanobiotechnology at the same department and is scheduled to be done in 2017. His research interests are among others drug delivery systems, enzyme optimisation and electrospinning.

P. Fojan received his Ph.D. in Biotechnology at the University of technology Graz, Austria in 1997. He initially worked on industrial genetics of eukaryotic organisms. During his postdoc time at Aalborg University at the Department of Biotechnology he moved into the area of protein physics and molecular modelling. With the startup of Nanotechnology at AAU he moved to the Department of Physics and Nanotechnology where he became an Associate Professor in 2009. His research interests are centered around biological and small molecules and their interactions with cells and surfaces in general, for medical, sensor applications and as antibacterial agents.
Journal of Self-Assembly and Molecular Electronics, Vol. 1, 17–30.
doi: 10.13052/jsame2245-4551.2017.002
© 2017 River Publishers. All rights reserved.