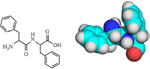The Many Faces of Diphenylalanine
Received 18 June 2013; Accepted 06 August 2013; Publication 6 August 2013
Journal of Self-Assembly and Molecular Electronics, Vol. 1, 195–208.
doi: 10.13052/jsame2245-4551.123
Copyright © 2013 River Publishers. All rights reserved.
Mohtadin Hashemi, Peter Fojan and Leonid Gurevich*
- Institute of Physics and Nanotechnology, Aalborg University, 9220 Aalborg East, Denmark
- *Corresponding author: lg@nano.aau.dk
Abstract
Diphenylalanine is well known to form complex self-assembled structures, including peptide nanowires, with morphologies depending on N- and C-terminal modifications. Here we report that significant morphological variations of self-assembled structures are attainable through pH variation of unmodified diphenylalanine in trifluoroethanol. The obtained self-assembled diphenylalanine nanostructures are found to vary drastically with pH, incubation time, and diphenylalanine concentration in solution. The observed structures ranged from structured films at neutral and alkaline conditions to vertically aligned nanowires and sponge-like structures at acidic conditions. These observations are corroborated by the results of electrostatic modelling, indicating the disappearance of the dipole moment at high pH values. This also emphasizes the importance of the dipole moment for the resulting self-assembled structures. Our results suggest that, in comparison to the commonly described procedure of diphenylaniline nanowire growth through aniline vapor treatment, strictly anhydrous conditions are not necessarily required.
Keywords
- Diphenylalanine
- peptide self-assembly
- peptide nanowires
- peptide nanotubes
1. Introduction
Self-assembly is a promising route to form functional nanostructured materials. In particular, peptides are specifically designed by Nature to self-assemble into complex structures [1]. This makes peptides a viable building material, in particular when biocompatibility is required [2–4]. Another major technological advantage of peptides is the well-known coupling chemistry and the diversity of chemical and physical properties of individual amino acids leading to a vast number of possible combinations [5].
From a different perspective, although biological functions have been generally ascribed to proteins, a vast number of biologically active short peptides have been characterized in the past with functions ranging from storage and transport to antimicrobial activity [6–9] and toxins [10, 11]. Recently, short peptides have been linked to a number of neurodegenerative disorders such as Alzheimer’s, Parkinson’s, and Prion diseases [12–14]. Studies have revealed that these conditions are related to peptide self-assembly properties, commonly known as amyloidogenesis. During this process a soluble and innocuous peptide turns into insoluble aggregates forming amyloid fibrils [14–16].
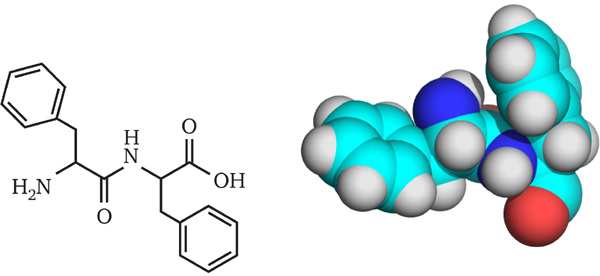
The search for a model system to study the mechanism of amyloid formation led to the shortest possible self-assembling peptide consisting of just two units – diphenylalanine (FF) (Figure 1)[17, 18]. Further studies have demonstrated that FF can assemble into a variety of structures including peptide nanotubes (PNTs) [19].
Figure 1 2D and 3D (CPK model) structure of diphenylalanine.
The role of electrostatic interaction between FF units during PNT growth has been investigated using FF with differently modified termini, which have been found to form vertically aligned tubes, non-aligned tubes, or only a minimal amount of tubes [19].
Another route for the formation of FF peptide nanowires (PNW) is based on aniline vapor treatment of FF amorphous films at elevated temperatures [20, 21]. Furthermore, PNWs can serve as templates for the formation of more complex structures for various applications, e.g. PNW/polyaniline core/shell [22], coaxial nanocables [23], stencil masks for nanolithography [24], and electrochemical sensors [25–27] have been demonstrated.
In the present paper we explore the possibility to affect the morphology of self-assembled structures by tuning the charge on the peptide via pH adjustment. We report the formation of various self-assembled structures using solutions of FF in TFE at different pH conditions and different incubation times.
2. Experimental Procedures
2.1. Synthesis of Diphenylalanine
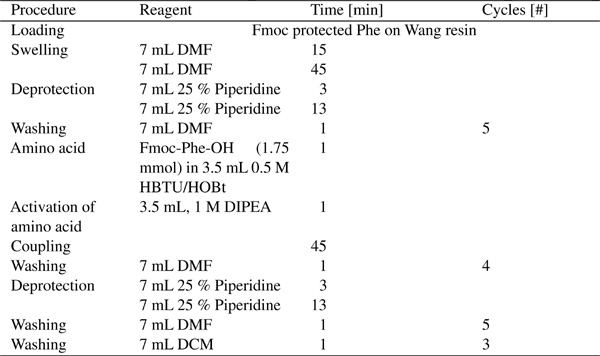
Diphenylalanine was synthesized ( theoretical yield) using SPPS on an Activo P11 Automated Peptide Synthesizer (Activotec, UK) following the protocol described in Table 1.
Table 1 Solid phase peptide synthesis protocol
DIPEA (N,N’-diisopropylethylamine), HBTU (O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate), piperidine, and Fmoc-Phe-OH were acquired from Advanced Chemtech. Phe on Wang resin (), HOBt (N-hydroxybenzotriazole), DCM (dichloromethane), and DMF (N,N1’-dimethylformamide) were obtained from Iris Biotech GmbH.
Cleavage
Removal of side-chain protection groups and cleavage of FF from Wang resin was achieved by 90 min incubation in solution, consisting of TFA (trifluoroacetic acid) (Iris Biotech GmbH), deionized water, and TIS (triisopropylsilane) (Fluka). After cleavage, FF in TFA/TIS was upcon-centrated in a rotary evaporator and subsequently precipitated using ice-cold diethylether (Iris Biotech GmbH). The obtained FF powder was dissolved in TFA and freeze dried.
2.2. Self-assembly of Diphenylalanine
Lyophilized FF was dissolved in TFE (Fluka), and ultrasonicated for 1 min following the initial disolution step. For pH adjustment, either NaOH (Bie & Berntsen) or HCl (Fluka) was added to of the FF solution. The FF solution was equilibrated at ambient temperature for 5, 10, or 30 minutes. To avoid pre-aggregation, fresh stock solutions were prepared for each set of experiments.
Si-wafers coated with of thermally grown SiO2 (NOVA wafers) were cleaned by ultrasonication in 99% ethanol (Kemetyl), rinsed and dried under a stream of N2.
For self-assembly, a droplet of FF solution was deposited on the Si-wafer and allowed to completely evaporate at ambient temperature.
To grow PNWs, self-assembled peptide films on Si-substrates were vapor treated with aniline (Sigma-Aldrich) in vacuum at .
2.3. SEM
Samples were imaged using a Zeiss EVO 60 with an acceleration voltage of and samples used “as is ”, unless otherwise stated. If coating was necessary, the sample has been sputter coated with Au for 30 seconds using Edwards S150B sputter coater.
2.4. Electrostatic Map
The diphenylalanine structure obtained by energy minimization (steepest descent and Newton-Raphson) was fed to the Kryptonite PDB2PQR server [28], with application of PROPKA [29] for pKa calculations. Adaptive Poisson-Boltzmann Solver (APBS) [30] was used to calculate the electrostatic potential using the linearized Poisson-Boltzmann equation. Parameters, except pH, for Kryptonite server and APBS were kept at default values. To account for the presence of residual water in TFE, the dielectric constant was set to 10.
3. Results & Discussion
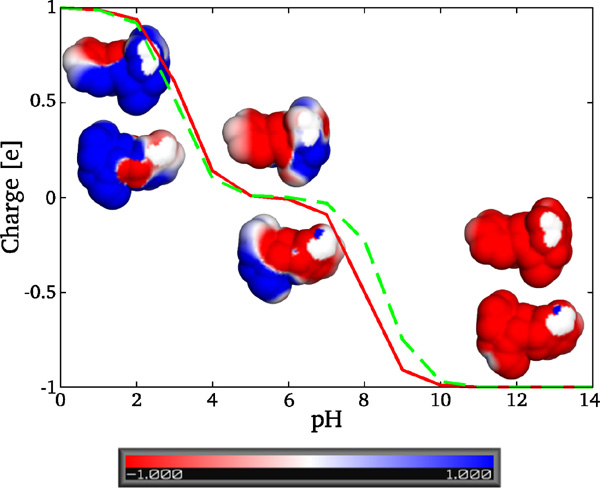
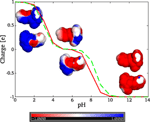
Elecrostatic modelling of the diphenylalanine peptide revealed three distinct states: at pH 0–2 the peptide is cationic, between pH 3–8 it is zwitterionic, and above pH 8 it is anionic (Figure 2).
Figure 2 Titration curve and electrostatic potential maps projected onto the solvent accessable surface of FF peptide at pH 0, 7, and 14. The colorbar is in units at . Solid line represents the charge of the folded state of FF, while dashed line corresponds to the unfolded state (states derived by PROPKA).
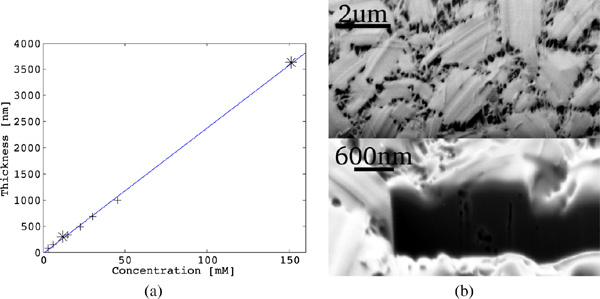
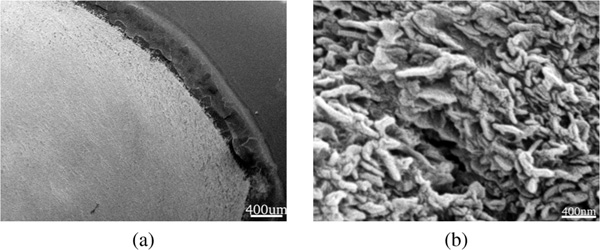
Diphenylalanine was found to initially form an amorphous film on the substrate, regardless of concentration or incubation time (Figure 3). The observed film thickness scaled linearly. Contrary to the claims of [22], stating that anhydrous environment is required to form film, we found that peptide film formed under ambient conditions and in Ar atmosphere as well.
Figure 3 (a) Thickness of the peptide film formed on the substrate under ambient conditions plotted versus FF concentration. Data from the present study denoted as *, data from [21] marked as +. The obtained relation is linear with the best fit , where the FF concentration is expressed in and the thickness d is obtained in . (b) SEM image of the peptide film formed in ambient environment, using FF in TFE and incubation. Bottom shows peptide film milled with FIB in situ. Images taken with Zeiss 1540 XB with acceleration voltage of .
According to literature the peptide film is believed to be amorphous [31], however in our case the film was not a continuous entity, but rather consisted of many individual, interconnected, micro-crystallites of typical size 2–3 (Figure 3). The observed behavior of diphenylalanine is most probably related to solvent structuring during evaporation leading to the formation of microcrystallites. The network of interconnected micro-crystallites forms the observed peptide film. This is in good agreement with [32], where the peptide film has been found to be an assembly of large bundles of tubular structures arranged as spherulites.
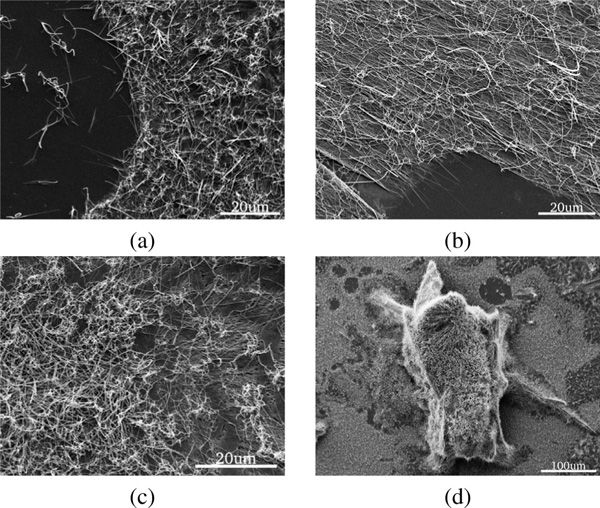
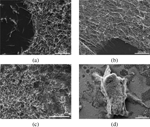
Incubation of the peptide film in aniline vapor at for yielded PNWs, with size and density depending on FF concentration (Figure 4). The peptide film, initially formed from a FF solution, underwent rearrangement from the amorphous state into individual PNWs (Figure 4.a) upon treatment with aniline vapor. No distinctive growth direction or size distribution was observed; the largest PNWs observed were in the range of 360–420 in diameter. Doubling the FF concentration to form the initial peptide film resulted in a similar behavior as described above, except for a higher wire density.
Figure 4 SEM images of different peptide films treated with aniline vapor at for . The precursor film was obtained using (a) , (b) , and (c) solutions of FF in TFE. (d)Zoom out showing a 3D island on peptide film from (c).
The peptide film formed from a solution with FF exhibited much higher density of PNWs as compared to the and samples. Besides that, the samples contained several islands (Figure 4.d and Figure 5) of vertically aligned wires. Preliminary results suggest that while formation of such 3D islands is independent of the incubation time in aniline vapor – the images obtained after 2, 4, and 8 hours incubation, all show similar structures (Figure 5). The total area of the islands formed was found to be time dependent.
Figure 5 (a) Zoomed image of the island in Figure 4.d showing vertically aligned PNWs. Peptide films were formed from FF solution and subsequently incubated in aniline vapor at for (b) 2, (c) 4, and (d) 8 hours.
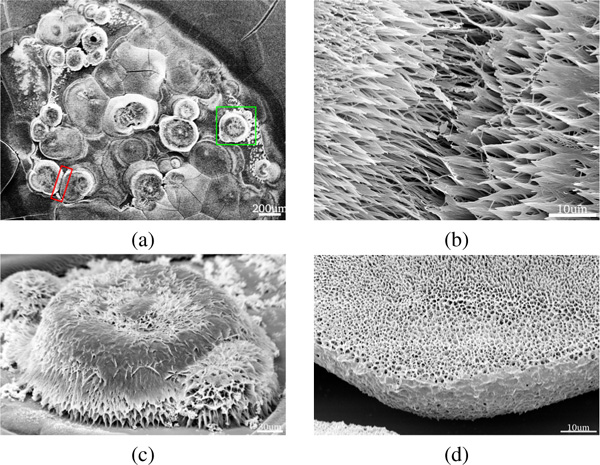
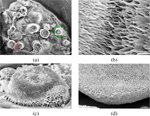
Acidic conditions, independent of incubation time, tend to favor the formation of 3D structures (Figure 6). The height profiles of the 3D structures varied between 90–130 for smaller structures and up to 210–270 for the largest structures observed. Incubation for 5 and 10 minutes yielded fibrous 3D “mushroom” structures (Figure 6.c). Upon extended incubation (), we observed merging of the fibrous 3D “mushroom” structures into porous “sponges” (Figure 6.d). These results suggest that the morphology of the 3D structures is incubation time dependent. Fibrous mushroom structures were found to form after incubation times of 5 and 10 minutes, while porous sponge-like structures require 30 minutes of incubation.
Figure 6 (a) SEM image showing 3D islands on an FF film formed from a FF solution under acidic conditions and incubation. The highlighted regions are magnified in panels (b) and (c). (d) Close-up of a sponge-like 3D structure formed after incubation.
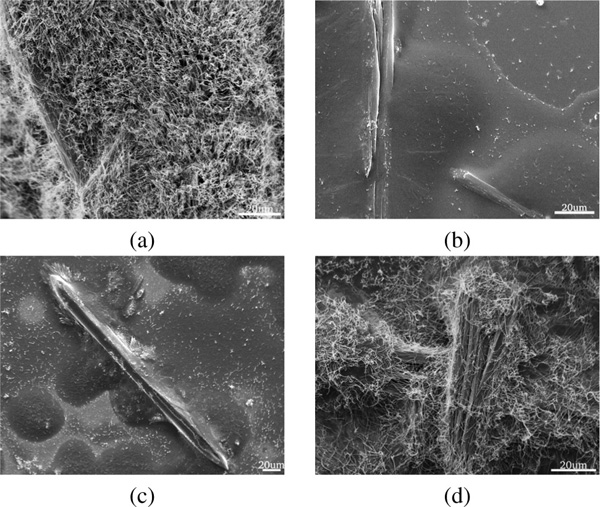
Structure formed under alkaline conditions had a drastically altered morphology (Figure 7). No PNW growth was observed and the structure consisted of small rod- and flake-like structures interwoven in a complex manner independent of the incubation time. This observation is in line with the electrostatic modelling results showing a lack of a distinct dipole moment at pH values above 8 indicating the importance of a dipole moment for structured self-assembly of diphenylalanine.
Figure 7 SEM images of self-assembled structures obtained under alkaline conditions from a FF solution deposited at ambient conditions. Panel (b) shows a zoom-in on the surface structure. Samples were coated with Au prior to SEM imaging.
4. Conclusion
All the reports on diphenylalanine self-assembly known in literature employed hexafluoro-2-propanol (HFIP) as a solvent. Moreover, strictly anhydrous conditions have been found to be necessary to achieve peptide nanowire growth upon aniline vapor treatment [20]. Our results suggest that a less hydrophobic solvent, trifluoroethanol, is capable of inducing the self-assembly of diphenylalanine. Moreover, by varying the pH of the trifluoroethanol solution of unmodified diphenylalanine, significant morphological variations were observed. While neutral and alkaline conditions favor formation of micro-structured films, vertically aligned nanowires and sponge-like structures were obtained at acidic conditions. This observation is in line with the results of [19], where a peptide with positively charged termini has been found to form well aligned peptide nanotubes, while negatively charged peptide have not formed any aligned structures. This has been attributed to the peptide interaction with a negatively charged silicon surface used as a substrate. In contrary to [19] we did not observe assembly into peptide nanowires at neutral pH, however this can be related to a slightly different protonation state of our substrate and peptide molecules as compared to [19]. Moreover, we found that using trifluoroethanol as a solvent did not require strictly anhydrous conditions to initiate the PNW growth through aniline vapor treatment. Similar results were observed in argon and ambient atmosphere. At lower concentrations the PNWs were observed to grow along the substrate, while at higher concentrations, islands of vertically aligned nanotubes started to appear. These results demonstrate a much wider parameter space for growing diphenylalanine nanowires and shows that pH can be used as an effective tool to control the morphology of peptide self-assembled structures [33].
Acknowledgements
The authors gratefully acknowledge financial support from the Obel Family Foundation.
References
[1] A. M. Kushner and Z. Guan. Modular Design in Natural and Biomimetic Soft Materials. Angew. Chem. Int. Ed., 50:9026–9057 (2011).
[2] L. Liu, K. Busuttil, S. Zhang, Y. Yang, C. Wang, F. Besenbachera and M. Dong. The role of self-assembling polypeptides in building nanomaterials. Phys. Chem. Chem. Phys., 13:17435–17444 (2011).
[3] R. de la Rica and H. Matsui. Applications of peptide and protein-based materials in bionanotechnology. Chem. Soc. Rev., 39:3499–3509 (2010).
[4] N. Srinivasan and S. Kumar. Ordered and disordered proteins as nanomaterial building blocks. WIREs Nanomed Nanobiotechnol, 4:204–218 (2012).
[5] R. B. Merrifield. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc., 85(14):2149–2154 (1963).
[6] T. Ganz. Defensins: Antimicrobial peptides of innate immunity. Nature Reviews Immunology, 3:710–720 (2003).
[7] E. Guani-Guerra, T. Santos-Mendoza, S. O. Lugo-Reyes and L. M. Teran. Antimicrobial peptides: General overview and clinical implications in human health and disease. Clinical Immunology, 135:1–11 (2010).
[8] G. Maroti, A. Kereszt, E. Kondorosi and P. Mergaert. Natural roles of antimicrobial peptides in microbes, plants and animals. Research in Microbiology, 162(4):363–374 (2011).
[9] M. Zasloff. Antimicrobial peptides of multicellular organisms. Nature, 415:389–395 (2002).
[10] H. Raghuraman and A. Chattopadhyay. Melittin: a Membrane-active Peptide with Diverse Functions. Biosci Rep, 27:189–223 (2007).
[11] G. Terlau and B. M. Olivera. Conus Venoms: A Rich Source of Novel Ion Channel-Targeted Peptides. Physiol Rev, 84:41–68 (2004).
[12] G. Bhak and Y.-J. Choe and S. R. Paik. Mechanism of amyloidogenesis: nucleation-dependent fibrillation versus double-concerted fibrillation. BMP reports, 42(9):541–551 (2009).
[13] J. A. Hardy and G. A. Higgins. Alzheimer's disease: The amyloid cascade hypothesis. Science, 256:184–185 (1992).
[14] M. Stefani, C. M. Dobson. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. Journal of Molecular Medicine, 81(11):678–699.
[15] J. W. Kelly. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr. Opin. Struct. Biol., 8(1):101–106 (1998).
[16] B. H. Toyama and J. S. Weissman. Amyloid Structure: Conformational Diversity and Consequences. Annu. Rev. Biochem., 80:557–85 (2011).
[17] A. T. Petkova, R. D. Leapman, Z. Guo, W.-M. Yau, M. P. Mattson, R. Tycko. Self-Propagating, Molecular-Level Polymorphism in Alzheimer's -Amyloid Fibrils. Science, 307:262–265 (2005).
[18] M. Reches and E. Gazit. Casting Metal Nanowires Within Discrete Self-Assembled Peptide Nanotubes. Science 300:625–627 (2003).
[19] M. Reches and E. Gazit. Controlled patterning of aligned self-assembled peptide nanotubes. Nature Nanotechnology, 1:195–200 (2006).
[20] J. Ryu and C. B. Park. High-Temperature Self-Assembly of Peptides into Vertically Well-Aligned Nanowires by Aniline Vapor. Advanced Materials, 20:3754–3758 (2008).
[21] J. Ryu and C. B. Park. Solid-Phase Growth of Nanostructures from Amorphous Peptide Thin Film: Effect of Water Activity and Temperature. Chemistry of Materials, 20:4284–4290 (2008).
[22] J. Ryu and C. B. Park. Synthesis of Diphenylalanine/Polyaniline Core/Shell Conducting Nanowires by Peptide Self-Assembly. Angew Chem Int Edit, 48:4820–4823 (2009).
[23] O. Carny, D. E. Shalev and E. Gazit. Fabrication of Coaxial Metal Nanocables Using a Self-assembled Peptide Nanotube Scaffold. Nano Letters, 6(8):1594–1597 (2006).
[24] M.B. Larsen, K.B. Andersen, W.E. Svendsen and J. Castillo-Leon. Self-Assembled Peptide Nanotubes as an Etching Material for the Rapid Fabrication of Silicon Wires. BioNanoSci., 1:31–37 (2011).
[25] I. De Oliveira Matos and W. A. Alves. Electrochemical Determination of Dopamine Based on Self-Assembled Peptide Nanostructure. ACS Appl. Mater. Interfaces 3:4437–4443 (2011).
[26] M. Yemini, M. Reches, J. Risphon and E. Gazit. Novel Electrochemical Biosensing Platform Using Self-assembled Peptide Nanotubes. Nano Letters, 5(1):183–186, 2005.
[27] J. Yuan, J. Chen, Z. Wu, K. Fang and L. Niu. A NADH biosensor based on diphenylalanine peptide/carbon nanotube nanocomposite. Journal of Electroanalytical Chemistry, 656:120–124 (2011).
[28] T. J. Dolinsky, J. E. Nielsen, J. A. McCammon and N. A. Baker. PDB2PQR: an automated pipeline for the setup, execution, and analysis of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Research, 32:W665-W667 (2004).
[29] M. H. M. Olsson, C. R. Sndergard, M. Rostkowski and J. H. Jensen. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa predictions. Journal of Chemical Theory and Computation, 7(2):525–537 (2011).
[30] N. A. Baker, D. Sept, S. Joseph, M. J. Holst and J. A. McCammon. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA, 98:10037–10041 (2001).
[31] J. Ryu and C. B. Park. High Stability of Self-Assembled Peptide Nanowires Against Thermal, Chemical, and Proteolytic Attacks. Biotechnol Bioeng, 105(2):221–230 (2010).
[32] N. Hendler, N. Sidelman, M. Reches, E. Gazit, Y. Rosenberg and S. Richter. Formation of Well-Organized Self-Assembled Films from Peptide Nanotubes. Advanced Materials, 19:1485–1488 (2007).
[33] L. Gurevich, T. W. Poulsen, O. Z. Andersen, N. L. Kildeby and P. Fojan. PH-dependent self-assembly of the short Surfactant-like peptide KA6. J. Nanosci. Nanotechnol., 10:7946–7950 (2010).
Biographies

Mohtadin Hashemi received his Master’s degree in Nanobiotechnology from the Institute of Physics and Nanotechnology, Aalborg University in 2013. His research interests are focused on protein interactions, molecular modelling and functional surfaces.

Peter Fojan received his Ph.D. in Biotechnology at the University of technology Graz, Austria in 1997. He initially worked on industrial genetics of eukaryotic organisms. During his postdoc time at Aalborg University at the Department of Biotechnology he moved into the area of protein physics and molecular modelling. With the startup of Nanotechnology at AAU he moved to the Department of Physics and Nanotechnology where he became an Associate Professor in 2009. His research interests are centered around biological and small molecules and their interactions with cells and surfaces in general, for medical, sensor applicationsand as antibacterial agents.

Leonid Gurevich received his Ph.D. in Physics at the Institute of Solid State Physics (Chernogolovka, Russia) in 1994. He initially worked on high-Tc superconductors but during his postdoc stay at Delft University of Technology became excited about nanotechnology and possibility of charge transport through a single molecule. Since 2005 he is an Associate Professor at Aalborg University. His research interests are focused on molecular electronics, biosensors, functional surfaces and nanofabrication.