Gas-phase Ion Spectroscopy of Flexible and Nonflexible Nitrophenolates: Effect of Locking the Two Phenyl Units in 4’-nitro-[1,1’-biphenyl]-4-olate by a Bridging Atom
DOI:
https://doi.org/10.13052/jsame2245-4551.6.001Keywords:
Intrinsic electronic absorption, charge transfer, nitrophenolates, mass spectroscopy.Abstract
Nitrophenolates (NPs) are molecular anions that can undergo charge-transfer
(CT) transitions determined by the degree of electron delocalization between
the phenolate oxygen (donor group) and the nitro group (acceptor). Here
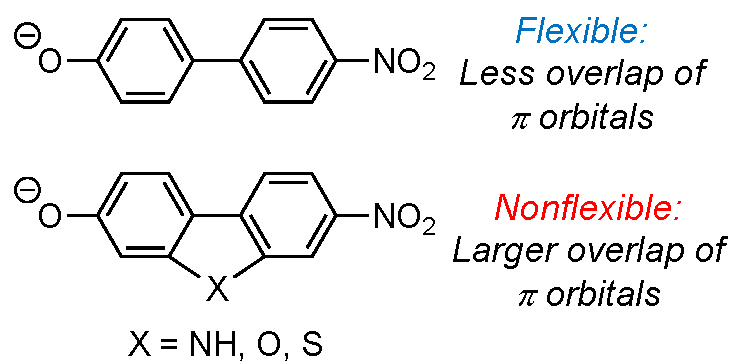
we have studied four different NPs: 4’-nitro-[1,1’-biphenyl]-4-olate (1),
7-nitro-9H -carbazol-2-olate (NH linker, 2), 7-nitrodibenzo[b,d]furan-3-
olate (oxygen linker, 3), and 7-nitrodibenzo[b,d]thiophen-3-olate (sulphur
linker, 4), and recorded their electronic absorption spectra when isolated
in vacuo to determine the effect of locking the biphenyl spacer group between
the donor and acceptor on transition energies. Absorption was identified from
ion dissociation (action spectroscopy) using a homebuilt setup (sector mass
spectrometer combined with pulsed laser). We find that the absorption is
broad in the visible region for all four NPs with significant vibronic features.
The lowest energy peak is at 601 ± 4 nm, 606 ± 4 nm, 615 ± 4 nm, and
620 ± 4 nm, for 3, 4, 2, and 1, respectively. NP 1 is flexible, and its lowest
energy structure is nonplanar while the other three NPs are planar according
to density functional theory calculations. Hence in the case of 1 the electronic transition has a higher degree of CT than for the other three, accounting for its
absorption furthest to the red. Our work demonstrates that oxygen and sulphur
are best at conveying the electronic coupling between the donor and acceptor
sites as 3 and 4 absorb furthest to the blue (i.e., the degree of CT is lowest
for these two NPs). Based on the average spacing between the peaks in the
vibrational progressions, coupling occurs to skeleton vibrational modes with
frequencies of 649 ± 69 cm−1 (3), 655 ± 49 cm−1 (4), and 697 ± 52 cm−1 (2).
Downloads
References
M. J. Cho, D. H. Choi, P. A. Sullivan, A. J. P. Akelaitis, L. R. Dalton,
Prog. Polym. Sci., 33, 1013–1058 (2008).
I. Alata, C. Dedonder, M. Broquier, E. Marceca, C. Jouvet, J. Am. Chem.
Soc., 132, 17483–17489 (2010).
D. Vonlanthen, A. Mishchenko, M. Elbing, M. Neuburger,
T. Wandlowski, M. Mayor, Angew. Chem. Int. Ed., 48, 8886–8890 (2009).
a) K. Dimroth, A. Schweig, C. Reichardt, Justus Liebigs Ann. Chem., 669,
–105 (1963); b) C. Reichardt, Chem. Rev., 94, 2319–2358 (1994).
E. M. Kosower, J. Am. Chem. Soc., 80, 3253–3260 (1958).
M. J. Kamlet, J. L. Abboud, R. W. Taft, J. Am. Chem. Soc., 99, 6027–6038
(1977).
a) M. J. Kamlet, R. W. Taft, J. Am. Chem. Soc., 98, 377–383 (1976);
b) R. W. Taft, M. J. Kamlet, J. Am. Chem. Soc., 98, 2886–2894 (1976).
S. Brøndsted Nielsen, M. Brøndsted Nielsen, A. Rubio, Acc. Chem. Res.,
, 1417–1425 (2014).
M.-B. S. Kirketerp, M. Å. Petersen, M. Wanko, L. A. E. Leal, H.
Zettergren, F. M. Raymo, A. Rubio, M. Brøndsted Nielsen, S. Brøndsted
Nielsen, Chem. Phys. Chem., 10, 1207–1209 (2009).
J. Houmøller, M. Wanko, A. Rubio, S. Brøndsted Nielsen, J. Phys. Chem.
A, 119, 11498–11503 (2015).
K. Støchkel, B. F. Milne, S. Brøndsted Nielsen, J. Phys. Chem. A, 115,
–2159 (2011).
J. A. Wyer, S. Brøndsted Nielsen, Angew. Chem. Int. Ed., 51,
–10260 (2012).
T. H. Jepsen, M. Jørgensen, M. B. Nielsen, Synthesis, 45, 1115–1120
(2013).
Gaussian 03, Revision D.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel,
G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr.,
Bjarke Møller Pedersen and Steen Brøndsted Nielsen
T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar,
J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega,
G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda,
J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai,
M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken,
C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J.
Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma,
G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich,
A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck,
K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul,
S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu,A. Liashenko, P. Piskorz,
I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y.
Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson,
W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian, Inc.,
Wallingford CT, 2004