In Situ Atomic Force Microscopy Studies of the Effect of Indolicidin on E.coli Cells
DOI:
https://doi.org/10.13052/jsame2245-4551.6.002Keywords:
Atomic force microscopy, indolicidin, antimicrobial peptides, E.coli cells, gelatin, honeycomb pattern, PLA, intracellular killing mechanism.Abstract
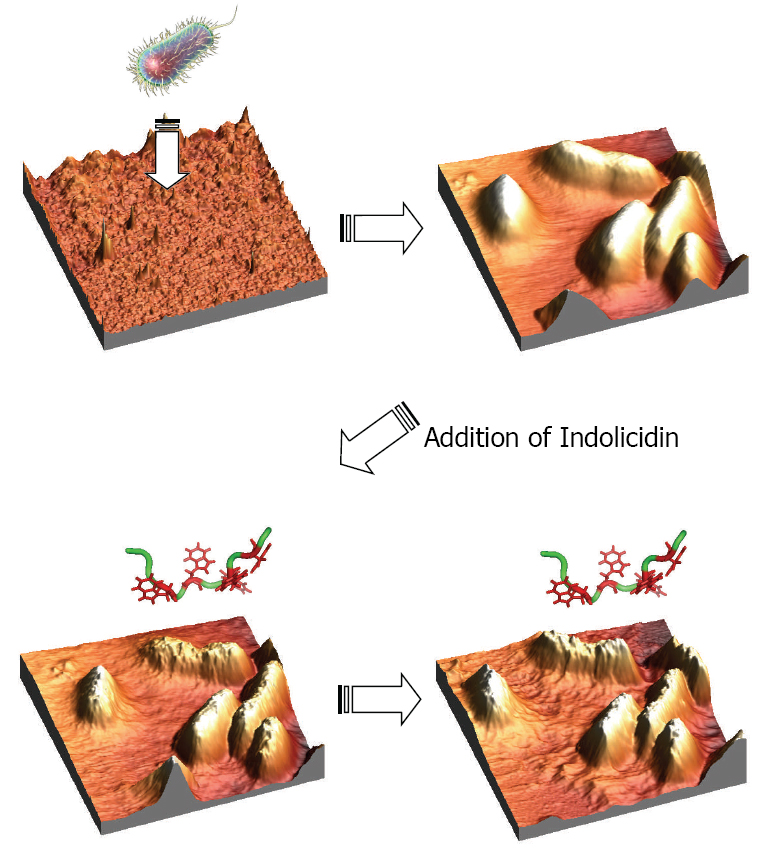
E.coli cells were succesfully attached to both gelatin coated surfaces and
polylactic acid honeycomb patterned mica surfaces as determined by in situ
atomic force microscopy. The gelatin coated surfaces provided a softer support
onto which the E.coli cells were capable of slightly submerging leading to a
better adhesion compared to the harder surfaces consisting of polylactic acid
polymer surfaces. After continuous scanning in liquid media, the E.coli cells
remained rod shaped and smooth. Indolicidin, a 13-AA linear antimicrobial
peptide, was injected in order to visualize the peptide-membrane interac-
tions in real time. Instantly after the injection of the peptides, the bacterial
membranes were observed to be distorted and seemed to melt proceeding as a
function of time. In conclusion, these experiments proved that the E.coli cells
were not ruptured as could be expected due to pore formation and disruption
of the osmotic pressure. This indicates a possible intracellular target killing
mechanism of indolicidin interacting with E.coli cells.
Downloads
References
Dufrˆene, Y. F. (2004). Refining our perception of bacterial surfaces
with the atomic force microscope. Journal of bacteriology, 186(11),
–3285.
Dufrene, Y. F. (2001). Application of atomic force microscopy to micro-
bial surfaces: from reconstituted cell surface layers to living cells. Micron,
(2), 153–165.
Radmacher, M., Tillamnn, R. W., Fritz, M. and Gaub, H. E. (1992).
From molecules to cells: imaging soft samples with the atomic force
microscope. Science, 257(5078), 1900–1905.
Sullivan, C. J., Morrell, J. L., Allison, D. P. and Doktycz, M. J.
(2005). Mounting of Escherichia coli spheroplasts for AFM imaging.
Ultramicroscopy, 105(1–4), 96–102.
Doktycz, M. J., Sullivan, C. J., Hoyt, P. R., Pelletier, D. A., Wu, S.
and Allison, D. P. (2003). AFM imaging of bacteria in liquid media
immobilized on gelatin coated mica surfaces. Ultramicroscopy, 97(1–4),
–216.
Beckmann, M. A., Venkataraman, S., Doktycz, M. J., Nataro, J. P.,
Sullivan, C. J., Morrell-Falvey, J. L. and Allison, D. P. (2006). Measuring
cell surface elasticity on enteroaggregative Escherichia coli wild type and
dispersin mutant by AFM. Ultramicroscopy, 106(8–9), 695–702.
Boonaert, C. J. P., Rouxhet, P. G. and Dufrêne, Y. F. (2000). Surface
properties of microbial cells probed at the nanometre scale with atomic
force microscopy. Surface and Interface Analysis: An International
Journal devoted to the development and application of techniques for
the analysis of surfaces, interfaces and thin films, 30(1), 32–35.
Micic, M., Hu, D., Suh, Y. D., Newton, G., Romine, M. and Lu, H. P.
(2004). Correlated atomic force microscopy and fluorescence lifetime
imaging of live bacterial cells. Colloids and Surfaces B: Biointerfaces,
(4), 205–212.
Ge, G., Han, D., Lin, D., Chu, W., Sun, Y., Jiang, L. and Wang, C. (2007).
MAC mode atomic force microscopy studies of living samples, ranging
from cells to fresh tissue. Ultramicroscopy, 107(4–5), 299–307.
Ostendorf, F., Schmitz, C., Hirth, S., Kühnle, A., Kolodziej, J. J.
and Reichling, M. (2008). How flat is an air-cleaved mica surface?.
Nanotechnology, 19(30), 305705.
Missirlis, Y. F. and Katsikogianni, M. (2007). Theoretical and experimen-
tal approaches of bacteria-biomaterial interactions. Materialwissenschaft
H. J. Askou et al.
und Werkstofftechnik: Entwicklung, Fertigung, Pr ̈ufung, Eigenschaften
und Anwendungen technischer Werkstoffe, 38(12), 983–994.
Grantham, M. C., Dove, P. M. and Dichristina, T. J. (1997). Microbially
catalyzed dissolution of iron and aluminum oxyhydroxide mineral sur-
face coatings. Geochimica et Cosmochimica Acta, 61(21), 4467–4477.
Scheuerman, T. R., Camper, A. K. and Hamilton, M. A. (1998). Effects
of substratum topography on bacterial adhesion. Journal of colloid and
interface science, 208(1), 23–33.
Edwards, K. J. and Rutenberg,A. D. (2001). Microbial response to surface
microtopography: the role of metabolism in localized mineral dissolution.
Chemical Geology, 180(1–4), 19–32.
Katsikogianni, M. and Missirlis, Y. F. (2004). Concise review of mech-
anisms of bacterial adhesion to biomaterials and of techniques used in
estimating bacteria-material interactions. Eur Cell Mater, 8(3), 37–57.
Yao, X., Walter, J., Burke, S., Stewart, S., Jericho, M. H., Pink, D.
and Beveridge, T. J. (2002). Atomic force microscopy and theoretical
considerations of surface properties and turgor pressures of bacteria.
Colloids and Surfaces B: Biointerfaces, 23(2–3), 213–230.
Fukuhira, Y., Kitazono, E., Hayashi, T., Kaneko, H., Tanaka, M., Shimo-
mura, M. and Sumi, Y. (2006). Biodegradable honeycomb-patterned film
composed of poly (lactic acid) and dioleoylphosphatidylethanolamine.
Biomaterials, 27(9), 1797–1802.
Y. Fukuhira, H. Kaneko, M. Tanaka, M. Yamaga, S. Yamamoto, M. Shi-
monura, Colloids and Surfaces A: Physicochem. Eng. Aspects 313–314
(2008) 220.
Toke, O. (2005). Antimicrobial peptides: new candidates in the fight
against bacterial infections. Peptide Science: Original Research on
Biomolecules, 80(6), 717–735.
Boman, H. G. (2003). Antibacterial peptides: basic facts and emerging
concepts. Journal of internal medicine, 254(3), 197–215.
Hancock, R. E. and Scott, M. G. (2000). The role of antimicrobial peptides
in animal defenses. Proceedings of the national Academy of Sciences,
(16), 8856–8861.
Bradshaw, J. P. (2003). Cationic antimicrobial peptides. BioDrugs, 17(4),
–240.
Makovitzki,A.,Avrahami, D. and Shai, Y. (2006). Ultrashort antibacterial
and antifungal lipopeptides. Proceedings of the National Academy of
Sciences, 103(43), 15997–16002.
In Situ Atomic Force Microscopy Studies of the Effect of Indolicidin 31
Shai, Y. (2002). Mode of action of membrane active antimicrobial
peptides. Peptide Science: Original Research on Biomolecules, 66(4),
–248.
Sawyer, J. G., Martin, N. L. and Hancock, R. E. (1988). Interaction of
macrophage cationic proteins with the outer membrane of Pseudomonas
aeruginosa. Infection and immunity, 56(3), 693–698.
Subbalakshmi, C., Krishnakumari, V., Sitaram, N. and Nagaraj, R.
(1998). Interaction of indolicidin, a 13-residue peptide rich in trypto-
phan and proline and its analogues with model membranes. Journal of
biosciences, 23(1), 9–13.
Matanic, V. C. A. and Castilla, V. (2004). Antiviral activity of antimi-
crobial cationic peptides against Junin virus and herpes simplex virus.
International journal of antimicrobial agents, 23(4), 382–389.
Bera, A., Singh, S., Nagaraj, R. and Vaidya, T. (2003). Induction of
autophagic cell death in Leishmania donovani by antimicrobial peptides.
Molecular and biochemical parasitology, 127(1), 23–35.
Bhargava, A., Osusky, M., Hancock, R. E., Forward, B. S., Kay, W. W.
and Misra, S. (2007). Antiviral indolicidin variant peptides: Evaluation
for broad-spectrum disease resistance in transgenic Nicotiana tabacum.
Plant science, 172(3), 515–523.
Shaw, J. E., Alattia, J. R., Verity, J. E., Priv ́e, G. G. and Yip, C. M.
(2006). Mechanisms of antimicrobial peptide action: studies of indoli-
cidin assembly at model membrane interfaces by in situ atomic force
microscopy. Journal of structural biology, 154(1), 42–58.
Ramamoorthy, A., Thennarasu, S., Lee, D. K., Tan, A. and Maloy,
L. (2006). Solid-state NMR investigation of the membrane-disrupting
mechanism of antimicrobial peptides MSI-78 and MSI-594 derived from
magainin 2 and melittin. Biophysical journal, 91(1), 206–216.
Hsu, C. H., Chen, C., Jou, M. L., Lee, A. Y. L., Lin, Y. C., Yu, Y. P.
and Wu, S. H. (2005). Structural and DNA-binding studies on the bovine
antimicrobial peptide, indolicidin: evidence for multiple conformations
involved in binding to membranes and DNA. Nucleic acids research,
(13), 4053–4064.
Zasloff, M. (2002). Antimicrobial peptides of multicellular organisms.
nature, 415(6870), 389.
Chan, D. I., Prenner, E. J. and Vogel, H. J. (2006). Tryptophan-
and arginine-rich antimicrobial peptides: structures and mechanisms of
action. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1758(9),
–1202.
H. J. Askou et al.
Yau, W. M., Wimley, W. C., Gawrisch, K. and White, S. H. (1998). The
preference of tryptophan for membrane interfaces. Biochemistry, 37(42),
–14718.
Dougherty, D. A. (1996). Cation-p interactions in chemistry and biology:
a new view of benzene, Phe, Tyr, and Trp. Science, 271(5246), 163–168.
Schibli, D. J., Epand, R. F., Vogel, H. J. and Epand, R. M. (2002).
Tryptophan-rich antimicrobial peptides: comparative properties and
membrane interactions. Biochemistry and cell biology, 80(5), 667–677.
Askou, H. J., Jakobsen, R. N. and Fojan, P. (2008). An atomic force
microscopy study of the interactions between indolicidin and supported
planar bilayers. Journal of nanoscience and nanotechnology, 8(9),
–4369.
Horcas, I., Fern ́andez, R., Gomez-Rodriguez, J. M., Colchero, J. W. S.
X., G ́omez-Herrero, J. W. S. X. M. and Baro, A. M. (2007). WSXM: a
software for scanning probe microscopy and a tool for nanotechnology.
Review of scientific instruments, 78(1), 013705.
Bolshakova, A. V., Kiselyova, O. I., Filonov, A. S., Frolova, O. Y.,
Lyubchenko, Y. L. and Yaminsky, I. V. (2001). Comparative studies of
bacteria with an atomic force microscopy operating in different modes.
Ultramicroscopy, 86(1–2), 121–128.
Robichon, D., Girard, J. C., Cenatiempo, Y. and Cavellier, J. F. (1999).
Atomic force microscopy imaging of dried or living bacteria. Comptes
Rendus de l’Academie des Sciences-Series III-Sciences de la Vie, 322(8),
–693